Dentis' groundbreaking advancements in digital dentistry, strategic partnerships, and international growth strategies are propelling them to the forefront of dental innovation and market dominance.

One major shift in the Korean dental landscape is that it is becoming essential for clinics and hospitals to shorten processes, offer same-day treatment, and maximize their profits. Avoiding scheduling several appointments and reducing chair time enhances patient experiences, as well as attracting new clients such as dental tourists who have very limited available time. What are the main challenges with same-day treatment? How do your company products achieve this?
The prominent topic of discussion lately has been digital dentistry. Concerning same-day treatment, several crucial factors come into play, including intra-oral scanner software, milling, 3D printers, and materials for printed prosthetics, which constitute the final product. Currently, milling is available in the market, but there is a lack of developed materials for printed prosthetics. If a company succeeds in developing these materials for the digital industry, it will significantly enhance productivity while reducing costs for dentists. Success in materials development would undoubtedly be a game-changer in the market. While we endeavor to develop these materials, it's important to acknowledge that this process is both costly and time-consuming. Throughout various exhibitions, I've observed several German companies diligently working on the development of these materials, which are currently in the pipeline.
We anticipated the advent of the digital dentistry era. Back in 2015, we pioneered the development of SLA 3D printers and materials, resulting in our flagship product "Zenith," which holds the largest market share domestically. We are continually advancing this product. Recently, there has been substantial demand from dental clinics for large-scale 3D printers. Consequently, we developed the first 8K LCD 3D printers in Korea. Additionally, we have created materials for models, surgical guides, dentures, temporaries, splints, and printed clear aligners.
To further enhance our competitive advantage, I am actively pursuing collaborations with global partners such as Medit, based in Korea, and Chinese companies specializing in intraoral scanners and software.
While digital dentistry has been a focal point in the industry until now, I foresee a shift towards aesthetic dentistry. In anticipation of this aesthetic dentistry era, we launched Korea's first clear aligner business on a digital platform. To meet this trend, we acquired a software company called TNS as our affiliate. We introduced a digital platform called Serafin and another software named Seraview.
A crucial aspect of the clear aligner business is the clear brace sheet. Acquiring the patent for it has proven challenging, as Align Technology and ZenduraDental, based in the U.S., along with a German company, currently monopolize patents globally. However, our young researchers devised a groundbreaking idea to navigate around these patents, enabling us to register the patent domestically. We are in the process of registering the patent in Japan, with the possibility of acquisition a year later if no challenges arise. Material acquisition is vital for sustainability in the industry. Despite not being early adopters, we have set trends rather than merely following them. Moreover, I anticipate FDA approval in the latter half of this year, as our materials gain recognition. In fact, we recently secured 15.7 billion KRW in capital investments from venture capital for our affiliate TNS. This influx of capital will facilitate upgrading our production line, ensuring smoother global expansion.

Luvis M/L series
When discussing legacy companies, typically customers don't prioritize the product; instead, they place their trust in the practitioner. It's the practitioner whom you need to convince. However, when it comes to aligners, consumers are well-informed. Invisalign, for instance, enjoys significant recognition among consumers, not just practitioners. How do you intend to educate the market about your competitive edge compared to these established brands?
If we examine the shareholder composition of TNS, we find a significant presence of orthodontists. They take immense pride in their profession, having graduated from prestigious colleges and gained entry into highly competitive orthodontics departments. Consequently, they possess a profound level of clinical expertise and can cater to a wide array of customer needs. Yet, legacy brands may underestimate the Korean market, perceiving it as relatively small compared to the international market. However, the Korean industry is fiercely competitive, boasting the ability to complete processes within a mere two weeks and at a cost approximately 30% lower than that of Invisalign. Thus, assuming there are no issues with clinically verified materials, I firmly believe that the international market will turn its attention to Korea, drawn by our accumulated expertise and experience. Success seems inevitable for us, given our capacity to address diverse customer needs, unlike the less user-friendly Invisalign.
You've successfully developed the LED light system and the Luvis Chair, capturing a significant market share both domestically and in exports. Specifically, for these products, how were you able to acquire strong market share in the overseas regions? How do you compete against established brands like Morita in Japan?
A company must continuously adapt to evolving customer needs and technological advancements in the market. Initially focusing on implants, we boldly ventured into various other sectors, recognizing the potential synergy between implants and the manufacturing of dental equipment. In 2010, we embarked on developing LED dental lights, later expanding our portfolio to include other medical lights. We are the sole provider of both types of lights.
Although dental and medical industries are distinct, our pioneering spirit enabled us to establish a strong presence in both sectors. Attending exhibitions in both medical and dental fields, our primary aim has always been to become the premier company, offering cutting-edge technology and products. We've garnered recognition and built a reputation, with further growth anticipated in the near future. Notably, our Luvis products face competition from legacy brands in Germany and mid to low-cost brands, yet we're holding our own in this competitive landscape. Currently, our goal is to penetrate the U.S. market by leveraging exceptional logistics channels.
The brand power of Luvis is substantial, thanks to its breakthrough technology, commanding 90% of the dental market and 40% of the medical market. To capitalize on this success, we've expanded the Luvis lineup, naming products such as the Luvis Chair and Luvis ST500 to leverage the brand's global recognition.
In terms of distribution, we benefit from an extensive logistics network spanning 80 countries, facilitating the introduction of new products. Morita has a century-long history, but we view ourselves as a relatively new entrant in the chair business, having commenced just a year ago. While Morita is prominent in the Japanese domestic market, its international presence is not as robust. Similarly, Yoshida, another competitor, primarily focuses on the Japanese market. In contrast, Korean companies have positioned themselves as globally competitive, offering high-quality products at cost-effective prices. For instance, while a Morita chair costs approximately 40 million KRW, Korean products are priced at half that amount, making them more viable contenders on the global stage.

Luvis ST500
Dentis, established in 2005, has emerged as an innovative implant company in Korea. Transitioning to a new phase of development, Dentis now practices "total dentistry," providing comprehensive dental solutions, including implants, bone grafts, oral health products, and innovative digital dental equipment such as LED surgical lights and dental 3D printers. These represent treasures of Dentis' unique technological capabilities. As a total dentistry company, how important is it to be a total solution provider in order to satisfy the needs of your clients?
As I've previously mentioned, companies must evolve within the market. Korea presents a rather unique environment where implants have become accessible to the general population, unlike in other countries. To meet the diverse needs of our customers, it's imperative for companies to develop tailored products or lineups that can synergize with implant treatments.
Indeed, we initially specialized in implants, but we expanded our business into surgical lights, biomaterials such as bone grafts and membranes, and printing. This expansion was essential to complement implant procedures and enhance the convenience of clinical treatments for our customers. Additionally, we've developed products like Squva or intraosseous painless anesthetic devices to further cater to the needs of our clients.
You mentioned the importance of competition. How crucial is it to compete effectively in the Korean market? When we examine international companies like Invisalign and Morita, they are not as diversified as Dentis. Do you believe that the capabilities you've acquired in the Korean market will make you more agile and adaptable to spearhead new trends internationally?
Our products are currently gaining global recognition; however, obtaining approvals from regulatory bodies such as the FDA or CE entails stringent requirements. Once we secure FDA or CE approval, I anticipate substantial growth for our company. This is precisely why we've diversified our portfolio, with Denops, the Luvis Chair, and our bone materials all progressing towards obtaining approvals. Achieving these approvals will undoubtedly fuel our growth further. We're targeting the end of this year for obtaining CE approval and the end of next year for FDA approval for our bone materials. Additionally, Mesheet, our mesh-type clear brace sheet, is poised to receive FDA approval by June or within the third quarter.
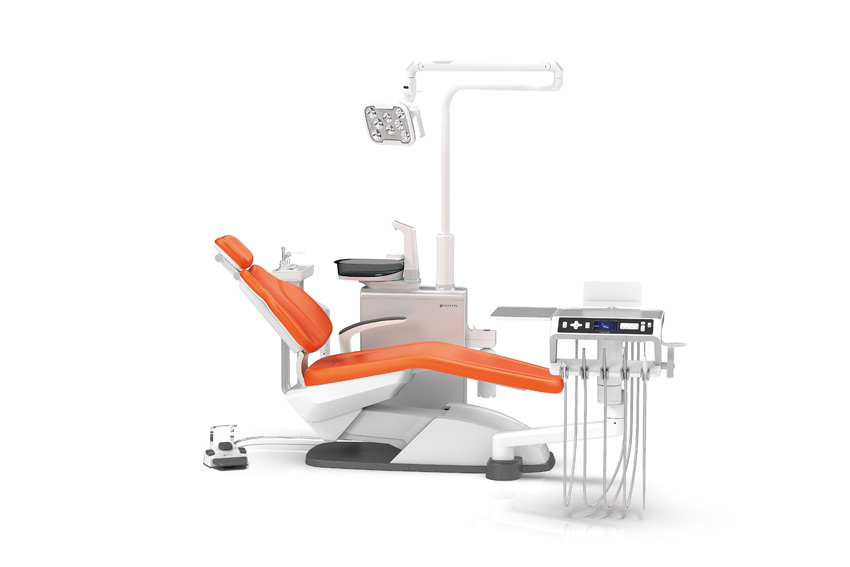
Luvis Chair
With more than 80 partners around the world and four corporations in Europe, China, and the US, Dentis has a strong positioning and revenue coming from the international market. However, Dentis’ internationalization is not only focused on sales, but it is multifaceted, as shown by the MOU signed with the German company Leica Microsystems for acquiring and integrating the M320 microscope into its portfolio, or the Dentis World Symposium mentioned previously. What is your international strategy? Are you actively seeking partners to develop new products and technologies?
I'd like to outline my primary goals for the upcoming year, as it will mark the 20th anniversary of our company's founding. We aim to conduct successful business through affiliates in three key areas: as a provider of comprehensive dental solutions, as a global clear aligner company in partnership with our affiliate TNS, and as a global provider of operating room solutions.
In terms of our implant business, we have established corporations in California, Seattle, and New Jersey in the U.S., as well as in Spain, Portugal, and China. Additionally, we plan to create corporations in India, Malaysia, and Poland. In the U.S., we're focused on expanding our branch network to meet nationwide demand, as the implant business requires direct sales.
The Luvis Chair is poised to obtain FDA approval by the end of March this year. Our objective is to identify outstanding B2B channels in the U.S. to distribute the Luvis Chair effectively. During an exhibition in San Francisco last November, where we showcased the Luvis Chair, we received positive feedback from American consumers due to its aesthetically pleasing design. This response was quite remarkable, as U.S. products typically emphasize pragmatism over aesthetics. The Luvis Chair, with its European-style elegance, resonated with consumers, offering both style and functionality.
Visiting the dentist can induce anxiety in patients, and traditional barriers or partitions can exacerbate this feeling. However, the floor-mounted design of the Luvis Chair enhances aesthetics and reduces patient anxiety, contributing to a more comfortable experience.
COVID-19 posed significant challenges for everyone, particularly for the field of dentistry. However, since 2020, Dentis has shown remarkable resilience in its financial performance. What can we anticipate from Dentis in the future? What are your aspirations, and which products do you believe will drive your success?
COVID-19 served as a catalyst for us to propel our business forward with renewed vigor. I believe we are well-prepared for the future. To enhance implant treatments, we've curated a product lineup aimed at improving both customer experience and medical practitioner efficiency. Our digital guidance system for implants is gaining recognition globally. Furthermore, compared to our initial state, we've garnered increased momentum for growth moving forward. We anticipate achieving a CAGR of 30% each year, fueled by the continuous addition of new products to our lineup, which will undoubtedly bolster our financial performance. Venture capitalists have also recognized the potential in our lineup, as evidenced by their substantial investments in Serafin and our clear aligners. While the past served as a springboard, I firmly believe that we are now poised for even greater growth momentum in the future.
For more details, explore their website at https://luvis.co.kr/eng/
0 COMMENTS