CG Bio, a biomaterial-focused company, delivers complete anti-aging solutions by offering materials for skin, cell, and bone regeneration, along with aesthetic products like fillers and PDO threads. The multifaceted 5-in-1 company is reshaping the US market by merging cost competitiveness with high-quality educational trainings for American surgeons.

South Korea is currently facing a unique demographic situation. With over 25% of the South Korean population expected to be over the age of 65, the nation is becoming one of the first super-aging societies in the world. However, this issue is not unique to Korea; other economies such as Japan, China, and the US are also grappling with the same challenge. What challenges and opportunities does this demographic situation present for medical companies?
Due to the aging population, we anticipate a sustained increase in demand for tissue regeneration and related sectors, particularly for bone and implant, bond, or cartilage repairs. Additionally, the anti-aging market is poised to grow as individuals seek ways to maintain a youthful appearance. This trend will positively impact the aesthetics and dermal filler market, leading to an increased demand for such products. Simultaneously, governments are grappling with the financial strain of rising medical expenses, prompting a greater emphasis on cost-effective medicines. Consequently, there is a growing preference for high-quality products that are also economically priced. In this context, CG Bio and the broader medical sector are strategically positioning themselves to target the aging population through regenerative medicine while concurrently prioritizing cost-effectiveness.
In 2021, the medical device market in South Korea reached a market size of 9.1 trillion KRW. However, critics, such as Professor Sun Kyong of Kyung Hee University, argue that this growth is primarily attributed to foreign companies rather than domestic ones. How do you foresee this trend evolving, and what strategies can Korean companies employ to reclaim domestic market shares?
To illustrate this point, let's consider prominent companies like Samsung, LG, and Hyundai Motor Group. Two decades ago, when I founded my own company, South Korea's medical sector primarily focused on class one or class two devices. However, for class three and class four, such as implantable devices, we were reliant on 100% imported medical devices. Today, South Korea boasts mass production technology, signaling a concerted effort to further localize technologies.
Nevertheless, a challenge persists as South Korea continues to import cutting-edge medical devices from overseas. However, the Korean government is actively shifting its approach, aiming to localize select markets. Cost-effective products are a focal point for many Korean companies, striving to achieve mass production in this category. Companies like Samsung, LG, and Hyundai Motor Group, while offering relatively lower prices compared to competitors, maintain a higher standard of quality than counterparts in China. Although innovation remains a challenge, Korea's advancements in information technology have prompted the government to promote AI and robotics for digital healthcare. This strategic focus aligns with the direction of CG Bio as well.
Korean medical equipment is known for its dual strengths in cost-effectiveness and innovation. However, a notable challenge arises, particularly in countries like Japan and America, where hospitals and medical centers tend to be conservative. Convincing them to transition to a new brand or adopt innovative equipment can be challenging. Despite this, success stories in Korea, such as Inbody and Osstem, have demonstrated significant international growth. How crucial are international markets for companies like CG Bio, and what strategies can firms employ to enhance Korea's reputation and overcome conservatism on a global scale?
Drawing from my firsthand experience, the South Korean market constitutes a mere 1.5% of the global market, making it imperative for us to explore opportunities overseas. Yet, being in Korea has its merits. The widespread health insurance coverage among South Koreans creates an environment that compels us to consistently develop cost-effective products. This challenging environment fosters continuous adaptation and modification of our medical devices, enhancing our competitiveness in the global export market.
In the U.S. and Japan, as you rightly pointed out, conservatism is particularly pronounced, especially concerning implants in the human body. This is where training and education play a pivotal role. Osstem's success, for instance, was underpinned by a strategy that prioritized education, training, and subsequently, sales. South Korean doctors and surgeons provide hands-on experience through workshops, a fast-track approach to growth. Merely offering good products is insufficient if surgeons are unfamiliar with their usage. Surgeon-to-surgeon education is paramount. Consequently, CG Bio has established branches overseas, where our strategy involves connecting local surgeons with their Korean counterparts who use our products, facilitating the exchange of experiences. This approach not only promotes education but also builds trust and familiarity, crucial elements in overcoming conservatism in the global arena.
Since its establishment in 2006, CG Bio has undergone substantial growth in the development and manufacturing of bone grafts, regenerative products, and an expanded range of solutions for surgical tissue reconstruction and wound management. Notably, the company has recently achieved significant success with its new offerings in aesthetic products, including fillers and PDO threads. Could you elaborate on the evolutionary journey of your company that led to its current business structure? Furthermore, how do the medical and aesthetic divisions collaborate to contribute to the overall growth of the company?
I frequently encounter this question, and it's a common inquiry from many. CG Bio might appear as though it has amalgamated five different companies, but in reality, we are a biomaterial-based company.
The materials utilized in crafting bone grafts can also be applied in the production of fillers. It all stems from the same technology centered around the regeneration of skin or bones. I want to emphasize that it's the identical principle and technology at play. Initially, our focus was on illnesses and treatments, specifically tissue regeneration—a fundamental principle that aligns with anti-aging objectives. The underlying logic for treating ailments, anti-aging, and cosmetics revolves around the regeneration of cells to create new tissue. In the case of treatment, it involves the regeneration of defective tissues or parts, which transforms into anti-aging technology when the same logic is applied to normal cells instead of defects. As the demand for anti-aging solutions grew, this technology naturally extended into the anti-aging sector. Essentially, it's the same tissue-regenerating technology, but the distinction lies in the target customers.
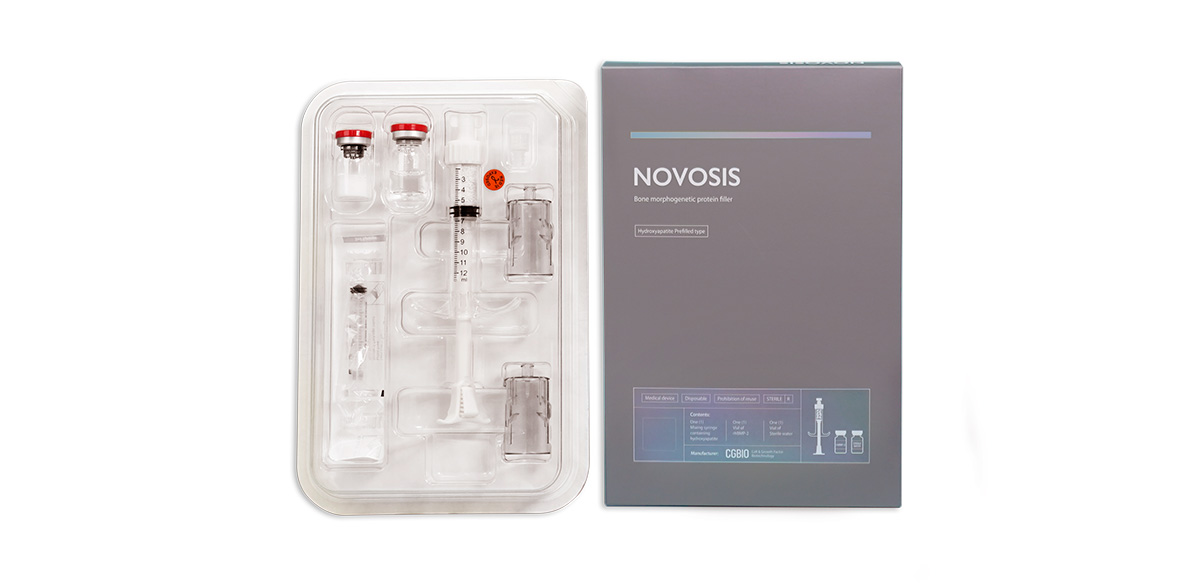
A bone substitute 'Novosis' incorporating the bone-promoting protein 'rhBMP-2' on a carrier made of Hydroxyapatite material.
Novosis is set to compete against the long-standing monopolistic bone graft product, Medtronic Infuse Bone Graft. How does Novosis intend to challenge Medtronic's monopoly, and what are your strategies for expanding market share in the field of bone grafting and regeneration?
Addressing your first question regarding why Novosis is superior to Infuse by Medtronic, the latter has held a market monopoly for the past two decades. Additionally, it releases BMP rapidly, in large amounts, posing a significant risk of side effects. This can lead to unintended bone regeneration and increase the likelihood of complications, including swelling and a potential link to cancer promotion. To overcome these issues, we focused on reducing the BMP dosage and implementing scaffolding technology for a gradual BMP release. As a result, Novosis utilizes only 25% of the BMP used by Infuse while maintaining comparable efficacy with fewer side effects, ensuring a safer alternative. Our expertise lies in slow-release technology, aligning with the current trend in the medical and pharmaceutical sector, specifically in drug delivery systems (DDS).
Personally, I specialize in artificial bones, and by using artificial bone as a scaffold, we achieve a gradual release of BMP, resulting in regenerated bones that are denser and stronger. These technological advancements are key advantages of our product.
Furthermore, in our commitment to reducing overall medical costs, we aim to facilitate quicker patient recovery post-surgery. Our approach involves utilizing BMP to stimulate new bone growth and developing a technology for minimally invasive surgery. Specifically, we are working on a spine endoscopy technique with a mere 1.5-centimeter incision for spinal fusion surgery. South Korean surgeons, known for their exceptional skills, can perform complex spinal surgeries through this small incision, drawing interest from surgeons globally, including in the U.S. Our goal is to standardize these surgical techniques, provide education and training to international surgeons, and enable patients to undergo surgery and return home on the same day, minimizing hospital stays. Similar to the U.S., where ambulatory surgical centers thrive, we aim to develop and expand our products and services, including spinal endoscopy technology, Novosis, and an expandable cage, facilitating swift patient treatment and discharge. This is our focal target.
Implementing minimally invasive surgeries presents a significant challenge, particularly in the area of surgeon training. While adopting new technologies is often feasible, the adaptation may vary across different markets. Could you provide more details about the training programs you offer and their significance within your overall strategy?
As previously mentioned, Korean spinal surgeons demonstrate exceptional proficiency in spinal endoscopy surgical techniques. However, the current challenge lies in the lack of standardization for these techniques. The absence of a standardized approach results in doctors using different techniques and devices, lacking a systemic framework. Our primary objective is to address this issue by standardizing all relevant technologies and devices, allowing uniform training for doctors. To achieve this, we have established a committee comprising doctors and relevant organizations with a shared interest in standardization. Through collaborative efforts, we are actively working to ensure that all doctors can receive standardized training and effectively utilize the devices. This commitment aligns with our broader strategy to facilitate widespread and accessible learning for medical professionals.
Recently, on top of having their own HA and Calcium Hydroxylapatite fillers, CG BIO has acquired DNC AESTHETICS, a Korean medical aesthetics product maker, and M.BASE, a lifting thread company, to further expand in the aesthetic surgery market. In addition, CG Bio signed a 100 million USD license out agreement in China for the FACETEM product. How does your background as a biotech company contribute to your expansion into the cosmetic industry? What are the key factors that contribute to your growth in this sector?
CG Bio specializes in injection-focused procedures, particularly in non-incision surgical operations such as using fillers to address wrinkles. For example, our calcium filler utilizes the same components as artificial bones. Hydroxylapatite, a material used in bone creation, when rounded, transforms into collagen, effectively reducing wrinkles. Leveraging my background with a Ph.D. in material engineering, we apply this knowledge to use hydroxylapatite for both bone grafting and collagen creation, operating on the same principle.
We utilize various biodegradable polymers to formulate different fillers tailored to specific needs. For instance, we develop epidermal growth factor-based fillers for collagen enhancement. Skin boosters are created by combining biologically active substances like DNA, PDRN, and other polymers. Stem cells, extracted from a person's own fat, are cultured and then injected into the face to enhance skin elasticity. The use of PDO, a biodegradable polymer, in filler manufacturing goes beyond wrinkle improvement; our aim is to restore skin conditions to their youthful state. The core focus is on utilizing biodegradable polymers to stimulate collagen regeneration, driving our expansion into the cosmetics industry.
It's crucial to note that the effectiveness of cosmetic products is limited if applied directly to the face due to the thickness of the skin barrier. To overcome this, we are actively developing micro needling technology, employing biodegradable polymers to further penetrate the cosmetics sector. Micro needles penetrate the skin, facilitating the spread of substances within the skin. This innovative approach aims to reduce freckles, lighten the skin, diminish pigmentation, and improve overall skin tone. This holistic strategy underscores our commitment to advancing in the cosmetics industry with innovative and effective solutions.
Your business stands out as a hidden champion in Korea, experiencing rapid international expansion. Your medical devices have seen success in the U.S., China, and other countries, while your cosmetic business is rapidly growing in markets like China, Australia, and New Zealand. Could you elaborate on the key factors driving your continuous growth in the international market and shed light on your upcoming plans for the next five years?
The reference to a hidden champion is intriguing, especially considering the book by the German writer. I actually read that book in 2018 when I assumed control of the global division. The parallels between Korea and Germany in the book resonated with me. Both were small yet powerful countries, with Germany, much like Korea, being divided at one point. This division led to small domestic markets, pushing both nations to leverage strong technological capabilities and achieve growth by exporting cutting-edge products globally. I adopted a similar strategy for our company, aiming to use our hidden competitive products to penetrate markets worldwide—this became my globalization strategy. I firmly believe that our products are unique and competitive, serving as pivotal factors for global market entry. There's no other company globally offering products quite like ours. The differentiation and competitiveness inherent in our products attract interest, even without aggressive outreach or extensive marketing efforts. For instance, globally, only three manufacturers produce calcium dermal fillers, with CG Bio being one of them. This distinctive position prompts partners to seek us out proactively. As mentioned earlier, competitiveness and differentiation are paramount, and this approach, inspired by the insights from the Hidden Champion, has proven successful in our global business strategy.
Let's delve into the North American markets, which seem to be particularly promising for CG Bio. You recently established your U.S. presence, strategically located in close proximity to the FDA. With other facilities near customer centers, what are your expectations for the North American market in the years ahead, and how crucial do you believe this region will be for the growth of CG Bio?
Undoubtedly, the North American region holds immense significance as it constitutes 60% of the total global market. We firmly believe that it will be a decisive region for our growth. In the U.S., we are implementing diverse strategies, including partnerships and direct sales. The establishment of a U.S. branch serves two primary purposes. Firstly, it allows us to engage in direct marketing activities, and secondly, it provides a platform to acquire key technologies from the North American region. An interesting observation is that U.S. researchers often possess remarkable technologies but may lack experience in the commercialization of regenerative medicine. Leveraging my expertise in this area, we aim to create a synergy that benefits both parties. Additionally, high labor costs in the U.S. make products less cost-effective, leading us to conduct production in Korea, thereby ensuring more competitive pricing. Being in direct contact with American surgeons allows us to gather valuable insights, enabling us to tailor our products to meet the specific needs of U.S. doctors.
As you mentioned, our strategic location near the FDA serves to facilitate direct and smoother communication with the regulatory body, minimizing potential hurdles and expediting approvals. In summary, our focus on the North American market is driven by three key objectives: acquiring cutting-edge technology, engaging directly with U.S. surgeons for product refinement and marketing, and optimizing conditions for streamlined FDA approval processes. This multi-faceted approach positions us strategically for success in the North American market.
What is your ultimate goal, and what would you like to achieve during your tenure at CG Bio?
In the realm of medical devices for regenerative medicine, my aspiration for CG Bio is to attain a stellar reputation. I aim for CG Bio to be widely recognized—this is my ultimate goal.
For more details, explore their website at https://www.cgbio.co.kr/en/
0 COMMENTS